Program
Tuesday 25
- 10:00-10:45 Registration
- 10:45-11:00 Welcome
- 11:00-11:30 Roland Pellenq
- 11:30-12:00 Francois-Xavier Coudert
- 12:00-12:30 Jean Francois Dufreche
- 12:30-13:00 Amael Obliger
- 13:00-14:30 Lunch
- 14:30-15:00 Ignacio Pagonabarraga
- 15:00-15:30 Giovanni Ciccotti
- 15:30-16:00 Jure Dobnikar
- 16:00-16:30 Coffee Break
- 16:30-17:00 Alessandro Siria
- 17:00-17:30 Laurent Joly
- 17:30-18:00 Discussion
Wednesday 26
- 09:30-10:00 Alois Wurger
- 10:00-10:30 Signe Kjelstrup
- 10:30-11:00 Coffee Break
- 11:00-11:30 Guillaume Galiero
- 11:30-12:00 Fernando Bresme
- 12:00-12:30 Marisol Ripoll
- 12:30-14:00 Lunch
- 14:00-14:30 Carlos Nieto-Draghi
- 14:30-15:00 Samy Merabia
- 15:00-15:30 Bernard Rousseau
Abstract
Manipulating water and aqueous solutions with thermal gradients
Fernando Bresme
Computational Chemical Physics, Department of Chemistry, Imperial College London
Thermal gradients induce a number of coupling effects that are relevant in energy conversion applications, e.g., thermoelectric devices. It has been shown experimentally the possibility of generating large thermal gradients, 106 K/m and larger, by working at small scales (< micrometer). These studies raise interesting questions, both fundamental and practical, on how matter responds to such strong gradients.
In this talk I will discuss our current work on thermo-coupling effects in water and aqueous solutions under strong thermal gradients. Computer simulations provide a route to explore the response of water under thermal gradients and study non-equilibrium effects such as the thermal-polarisation of water, which needs to be considered in the extensions of non-equilibrium thermodynamic theories. I will show how the combination of computer simulations and non-equilibrium thermodynamics provides a route to determine the strength of these non-equilibrium coupling effects and to provide valuable microscopic insights into its physical origin.
Dynamical non-equilibrium Molecular Dynamics Approach to convection
Giovanni Ciccotti
Dipartimento di Fisica, Universita’ di Roma “La Sapienza”
We present the extension of standard equilibrium Molecular Dynamics to time-dependent nonequilibrium situations. We show how this is possible in full rigor by use of the nonlinear Kubo-Onsager relation, connecting dynamical nonequilibrium averages to equilibrium (initial distribution) averages over suitable time-evolved variables. To show the power of the method we apply it to study the onset of the convective circulation in liquids establishing as stationary asymptotic state convective rolls.
Theoretical insight into Soft Porous Crystals: Problems and tools to solve them
François-Xavier Coudert
CNRS & Chimie ParisTech, Paris, France
http://www.chimie-paristech.fr/molsim/
A large number of Metal–Organic Frameworks (MOFs) exhibit deformations of large amplitude induced by physical or chemical stimuli. These materials, termed Soft Porous Crystals, undergo swelling, pore opening/closing, and structural transitions under changes of temperature, mechanical stress, guest adsorption, electric fields, etc. They have attracted a lot of attention from materials scientists, who explore their potential practical applications (e.g. as sensors or nanoswitches) and try to engineer crystals with targeted properties.
Our team has built a “toolbox” of theoretical approaches to shed light into these materials properties, and in particular to address the interplay between the phenomena of adsorption, deformation and reactivity of these materials. Such tools include molecular simulation methods and algorithms for the understanding of the mechanical and hydrothermal stability of these materials, as well as thermodynamic models to understand the adsorption-induced structural transitions, and the coadsorption and separation properties of Soft Porous Crystals. We strive to use these various methods to provide a coherent description of Soft Porous Crystals from the unit cell scale all the way to the behavior of the whole crystal.
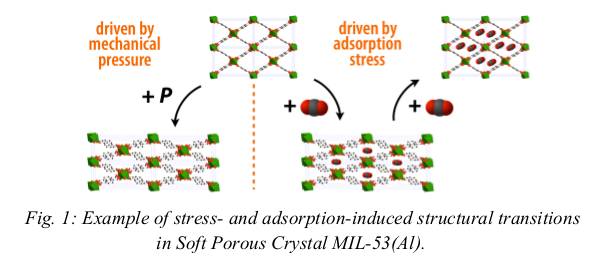
- [1] S. Horike, S. Shimomura, S. Kitagawa, Nature Chem. 2009, 1, 695.
- [2] A. Ortiz, A. Boutin, A.H. Fuchs, F.-X. Coudert, Phys. Rev. Lett. 2012, 109, 195502.
- [3] F.-X. Coudert, C. Mellot-Draznieks, A.H. Fuchs, A. Boutin, , J. Am. Chem. Soc. 2009, 131, 11329.
Stimulus-sensitive colloidal walkers
Jure Dobnikar
Colloidal particles with DNA “legs” that can bind reversibly to receptors on a surface can be made to ‘walk’ if there is a gradient in receptor concentration. We use a combination of theory and Monte Carlo simulations to explore how controllable parameters, e.g. coating density and binding strength, affect the dynamics of such colloids. We find that competition between thermodynamic and kinetic trends imply that there is an optimal value for both, the binding strength and the number of “legs” for which transport is fastest. Using available thermodynamic data on DNA binding, we indicate how directionally reversible, temperature-controlled transport of colloidal walkers can be achieved. The present results should make it possible to design a chromatographic technique that can be used to separate colloids with different DNA functionalization.
Multi-scale modeling of porous media for separation chemistry
J.-F. Dufreche, P. Turq, B. Siboulet, B. Coasne, O. Bernard, G. Allaire, A. Mikelic
Porous silica glasses are commonly used in the context of separation chemistry for dynamical separation methods. In that case, a solution for which some solute particles have to be removed goes through the glass and selective complexation groups grafted at the surface of the glass allow the separation.
This method is especially important for decontamination of heavy metals, such as radioactive elements and fission products in the context of nuclear energy. We used molecular modeling and multi-scale approaches in order to understand the properties of such systems and especially the dynamics of ions and molecules at the vicinity of silica surfaces.
The interactions and the dynamical processes strongly depends on the density of charged groups at the surface. After an irradiation the glass experimentally becomes more hydrophobic: even if the porosity is not modified, the Darcy coefficient is suddenly enhanced and the transport is going faster. Simulations show that this phenomenon can be related to the relaxation of the surface, which modifies the position of siloxane groups. Hydrophilic surfaces can be obtained with grafted silanol groups. Because of the rugosity, only a small amount of water molecules appear to be bounded to the surface.
The ion/surface interactions have also been examined. We show that Bjerrum electrostatic pairs are formed between the negatively charged oxygens of the silica and the cations of the solution. This phenomenon drives the electrostatic and transport properties in the system. Association constants depend of the nature of the ions and exhibit different values and Hofmeister series compared to the bulk results. An important part of the selectivity can be related to the size of the particles.
Then the macroscopic law have been calculated from a homogenization procédure. The departure to ideality have been taken into account thanks to the MSA theory of transport coefficients. We show that the macroscopic laws are not modified, but the values of the transport coefficients can be completely different.
From thermodiffusion to thermogravitation, a molecular simulation perspective.
Galliero Guillaume
Thermodiffusion is supposed to have a major effect on the initial vertical distributions of the species in a petroleum reservoir. In that frame, during the last dozen of years, we have studied this tricky transport phenomenon that couples heat and mass using non-equilibrium molecular dynamics simulations in various configurations. Among others, we will discuss about the influence of extreme confinement (nano-slit) on such a property in simple fluids. In addition, it will be shown that such molecular scale simulations can be employed under certain circumstances to prove the non-negligible influence on the vertical composition of typical reservoir fluids in a stationary 1D thermogravitational column.
Nanofluidic Osmotic Diodes: Theory and Molecular Dynamics Simulations
Laurent JOLY
Institut Lumière Matière - Université Lyon 1 (ljoly.ulyon@gmail.com)
Osmosis describes the flow of water across semipermeable membranes powered by the chemical free energy extracted from salinity gradients. While osmosis can be expressed in simple terms via the van ’t Hoff ideal gas formula for the osmotic pressure, it is a complex phenomenon taking its roots in the subtle interactions occurring at the scale of the membrane nanopores. Here we use new opportunities offered by nanofluidic systems to create an osmotic diode exhibiting asymmetric water flow under reversal of osmotic driving. We show that a surface charge asymmetry built on a nanochannel surface leads to nonlinear couplings between water flow and the ion dynamics, which are capable of water flow rectification. This phenomenon opens new opportunities for water purification and complex flow control in nanochannels.
The heat of transfer – an underestimated quantity
Signe Kjelstrup
Department of Chemistry, Norwegian University of Science and technology, Trondheim, Norway (signe.kjelstrup@ntnu.no)
On leave to: Institute of polymer physics, ETH, Zürich, Switzerland
In the study of thermal osmosis or the Soret effect, much of the attention has been devoted to understand the thermal diffusion coefficient, or the Soret coefficient. In this talk I argue that the reciprocal quantity, the heat of transfer is also interesting. It can provide a different perspective “on the same” as the two properties are related by the Onsager reciprocal relations. While the magnitude of the coupling coefficient for heat and mass fluxes is relatively small in homogeneous phases, it becomes non-negligible at interfaces. There is a large lack of data on these transport properties for interfaces. Examples will be discussed, taken from molecular dynamics - and other simulation studies.
On the importance of thermal boundary resistance in nanoscale heat transfer
Samy Merabia
Heat transfer across nanostructured materials is primarily controlled by the thermal boundary resistance between dissimilar materials, which is related to the phonon transmission coefficient [1,2]. Despite decades of research, our fundamental understanding of the physical processes at the origin of this resistance is quite poor. In this talk we will illustrate the flexibility of molecular dynamics simulations to probe the boundary resistance characterizing ideal interfaces, which in turn may help in understanding the basics of interfacial heat transfer [3]. In this perspective, we will quantifiy also the effect of the interfacial roughness on the change of the boundary resistance [4]. Finally, we will unveil the critical role played by the thermal boundary resistance in the vapor generation assisted by laser heated nanoparticles embedded in water [5].
References
- [1] E.T. Swartz and R.O Pohl, Rev. Mod. Phys. 61 605 (1989)
- [2] D.G. Cahill et al., JAP 93 793 (2002)
- [3] S. Merabia and K. Termentzidis, Phys. Rev B. 86, 094303 (2012)
- [4] S. Merabia and K. Termentzidis, Phys. Rev. B, 89 054309 (2014)
- [5] J. Lombard, T. Biben, S. Merabia, Phys. Rev. Lett., 112 105701 (2014)
Non-equilibrium molecular dynamics simulation techniques applied to CO2 capture and transport.
Carlos Nieto-Draghi
Thermodynamic and Molecular Simulation Department. IFP Energies nouvelles 1 et 4 avenue de Bois-Préau, 92852 Rueil-Malmaison Cedex - France
carlos.nieto@ifp.fr
The CO2 capture and sequestration (CCS) is an important challenge facing the scientific community today. It consists of a series of actions and processes involving the CO2 generated in different human activities. Three main steps can be summarized: 1) Capture, includes all possible industrial process (absorption, adsorption and membrane permeation) to capture CO2 at the emission points (i.e. Post combustion, pre-combustion, etc.), 2) Transportation, including the design of pipelines and storage facilities and 3) Sequestration, typically in deep saline aquifer reservoirs. The objective of the present work is to provide two examples of how thermodynamic/electric fields can be applied to study thermo-physical properties required in the capture and transport of CO2 by using non-equilibrium molecular dynamics (NEMD) simulation techniques. In the first case we test the concept of Nano electro-mechanical systems (NEMS) to induce reversible topological deformations of dielectric-functionalized hybrid organic-inorganic materials. We present preliminary NEMD simulations results using adapted integration algorithms[1] to explore the influence of the external electric fields on the material structure and its consequence on the diffusive patterns of ideal adsorbed particles. The second example is the prediction of the influence of gas impurities on the magnitude of the thermal conductivity of CO2 at dense conditions typically encountered in the pipelines and buffer storage facilities. The severe thermodynamic conditions (high pressures and relative high temperatures) as well as the presence of toxic impurities (such as H2S) make the experimental acquisition of thermal conductivity on such systems particularly difficult. We present boundary driven [2,3] NEMD simulation results of the thermal conductivity of CO2+N2, CO2+O2 and CO2+H2S mixtures at 130 bar and 150 bar at two temperatures of 288.15 and 293.15 K. The comparison of the simulation results with standard industrial correlation and equations of state is discussed to assess the limitation of the different approaches.
References :
- [1] Spreiter, Q.; Walter. M. J. of Comp. Phys. 1999, 152, 102-119.
- [2] Müller-Plathe, F. J. Chem. Phys. 1997, 106, 6082.
- [3] Nieto-Draghi, C.; Avalos, J. B. Mol. Phys. 2003, 101, 2303.
Pore network model of electrokinetic transportthrough charged porous media
A. Obliger 1,2,3, M. Jardat 1,2, D. Coelho 3, S. Bekri 4, B. Rotenberg 1,2.
1 Sorbonne Universités, UPMC Univ. Paris 06, UMR 8234 PHENIX, Paris, France
2 CNRS, UMR 8234 PHENIX, Paris, France
3 Andra, Châtenay-Malabry, France
4 IFP Energies nouvelles, Rueil-Malmaison, France
We introduce a method for the numerical determination of the steady-state response of complex charged porous media to pressure, salt concentration and electric potential gradients. The macroscopic fluxes of solvent, salt and charge are computed within the framework of a Pore Network Model (PNM) [1], which describes the pore structure of the samples as networks of pores connected to each other by channels. The PNM approach is used to capture the couplings between solvent and ionic flows which arise from the charge of the solid surfaces. The microscopic transport coefficients on the channel scale, taken here of a simple analytical form obtained previously by solving the Poisson-Nernst-Planck and Stokes equations in a cylindrical channel [2], are upscaled for a given network by imposing conservation laws for each pores, when macroscopic gradients are applied to the sample. The complex pore structure of the material is captured by the distribution of channel diameters. We investigate the combined effects of this complex geometry, the surface charge and the salt concentration on the macroscopic transport coefficients. The upscaled numerical model preserves the Onsager relations between the latter, as expected [3]. The calculated macroscopic coefficients behave qualitatively like their microscopic counterparts, except for the permeability (see Fig. 1) and the electro-osmotic coupling coefficient when the electrokinetic effects are strong. Quantitatively, the electrokinetic couplings increase the difference between the macroscopic coefficients and the microscopic ones for a single channel of average diameter.
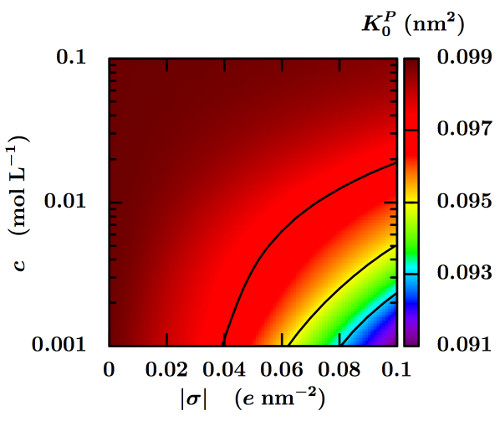
Macroscopic permeability K0P (in nm2) as a function of the salt concentration c in the reservoirs in equilibrium with the charged porous material, and of the surface charge density σ of the channels
References :
- 1. S. Békri, C. Laroche, O. Vizika. International Symposium of the Society of Core Analysts, 2005.
- 2. A. Obliger, M. Duvail, M. Jardat, D. Coelho, S. Békri, and B. Rotenberg, Physical Review E 88, 013019 2013.
- 3. E. Brunet and A. Ajdari, Physical Review E 69, 016306 2004.
Transport properties of alkanes in shale-rocks from the bottom-up approach
Roland Pellenq
"Multi-Scale Materials Science for Energy and Environment", <MSE>2
The CNRS and MIT joint laboratory, 77 Massachusetts Avenue Cambridge, 02139, MA, USA
Gas/oil shale are sedimentary rocks with ultralow permeability. The fundamental mechanisms controlling shale hydrocarbon extraction remain poorly understood, and the classic theories and simulation techniques used by the oil and gas industry have proven inadequate for shale source rocks. Flow through shale poses a distinctive challenge that is new to the oil and gas industry: a large part of pores in shale have typical widths in the order of a few angstroëms, and are within an organic porous material (kerogen) containing adsorbed hydrocarbons. At these scales, the pore size is on the order of the mean free path of the hydrocarbon molecules, and the Navier–Stokes equations with no-slip boundary condition cannot adequately represent the flow.
In the present talk, a generic model describing the flow in a multi-scale porous medium (such as shale) and fully taking into account the thermodynamics of confined fluids in nano and sub-nanopores, (including adsorption processes) is proposed without postulating any transport or diffusion mechanism. The model assumes that the rock pore void consist of different types of domains with different of pore sizes starting at the sub-nanometer level with a realistic atomistic description of the kerogen porosity (close to that of common porous carbons). This is a key improvement compared to current attempts to model flow (and production) in shale which all assume in the first place that the flow has to comply to Darcy’s behavior. Furthermore, our approach provides for the firm theoretical grounds for Archie’s law relating the in-situ flow to the porosity of the medium.
Implementation of temperature gradients in mesoscopic simulations
Marisol Ripoll
Theoretical Soft-Matter and Biophysics (ICS-2), Forschungszentrum Jülich, 52425 Jülich, Germany (m.ripoll@fz-juelich.de)
Multiparticle collision dynamics (MPC) is a simulation technique that provides a coarse- grained description of hydrodynamic fluids [1]. By construction, the MPC basic algorithm conserves mass, momentum, and kinetic energy, including also thermal fluctuations. Hybrid approaches have considered MPC to describe the solvent and molecular dynamic models to reproduce complex structures like colloids, polymers, or vesicles. A large number of these ap- proaches have already shown to be nicely suited to investigate systems where hydrodynamic interactions, and heat transport are relevant, for situations in and out of equilibrium.
The simulation of systems with temperature gradients relates to some specific aspects, which are present when using MPC, but not only restricted to it. One example is presence of a temperature jump between the temperature imposed in an specific area and the limiting temperature of the fluid. This temperature jump is also characteristic of experimental sys- tems and it depends on the fluid and the interface properties, as well as the system geom- etry [2,3,4]. In the determination of the thermophoretic properties of colloids, the presence of a thermophoretically induced flow and a corresponding back flow [5] has two relevant consequences. One is the very small differences between systems confined between walls at different temperatures, and open systems simulated with periodic boundary conditions together with temperature gradients of alternating direction. And two is that large finite size effects are intrinsic to the measurements of the colloid thermal diffusion factor [6].
- 1. A. Malevanets and R. Kapral, J. Chem. Phys., 110, 8605, (1999).
- 2. D. Lüsebrink and M. Ripoll, J. Chem. Phys., 136, 084106, (2012).
- 3. M. Yang and M. Ripoll, Phys. Rev. E, 84, 061401, (2011).
- 4. M. Yang and M. Ripoll, Soft Matter, 10, 1006, (2014).
- 5. M. Yang and M. Ripoll, Soft Matter, 9, 4661, (2013).
- 6. D. Lüsebrink, M. Yang and M. Ripoll, J. Phys.: Condens. Matter, 24, 284132, (2012).
Thermal Diffusion in simple fluid mixtures. What have we learnt from (NE) molecular dynamics simulations?
P.-A. Artola 1 and Bernard Rousseau 2
1. Université Paris Sud, Laboratoire de Chimie Physique, UMR 8000, 91405 Orsay, France
2. C.N.R.S., Université Paris Sud, Laboratoire de Chimie Physique, UMR 8000, 91405 Orsay, France
When a fluid mixture is subjected to a thermal gradient, it responds with concentration gradients: some species enrich at the cold side of the cell, while some others enrich at the hot side [1,2]. In binary liquid mixtures of chemically homologous molecules, heavier molecules usually go to the cold side. However, this simple rule is very fragile: a given species will flow in a certain direction according to its peculiar properties but also to temperature, pressure and most importantly, to the mixture composition! A typical example concerns water-ethanol fluid mixtures where water goes to the cold side at low concentration and to the hot side at high concentration. Recent experimental works have shown that thermal diffusion process is in many cases sensitive to molecular interactions between different species [3,4]. This was suspected from original works on transport properties and this partly explains why thermal diffusion phenomenon is so difficult to predict.
In this talk, we review what molecular dynamics has brought to the understanding of the thermal diffusion effect in simple fluid mixtures. We describe the various methods that have been developed by the community during the last decades to study this non-equilibrium phenomenon [5,6,7,8]. We present how these methods have been used to quantitatively predict transport coefficients. Finally, in the light of important advances in experimental setup that have brought accurate and reliable data in this area, we assess how molecular simulations contribute to the understanding of molecular mechanisms of the thermal diffusion effect and the elaboration of accurate macroscopic models.
References :
- [1] C. Ludwig, Sitz. ber. Akad. Wiss. Wien Math.-Nat. wiss. Kl 20 539 (1856)
- [2] C. Soret, C. R. Acad. Sci. 91 289 (1880)
- [3] C. Debuschewitz and W. Köhler, Physical Review Letters, 87, 55901 (2001)
- [4] B. J. de Gans, R. Kita, S. Wiegand and J. Luettmer-Strathmann, Phys. Rev. Lett. 91, 245501 (2003)
- [5] D. MacGowan and D. J. Evans, Physical Review A. 34, 2133 (1986)
- [6] G. V. Paolini and G. Ciccotti, Phys. Rev. A 35, 5156 (1987)
- [7] B. Hafskjold and T. Ikeshoji, S. K. Ratkje, Mol. Phys. 80, 1389 (1993)
- [8] D. Reith and F. Müller-Plathe, J. Chem. Phys. 112, 2436 (2000)
Nanofluidics to channel the osmotic energy
Alessandro Siria
Osmotic power refers to the free energy that can be extracted from the difference in salinity between salty and fresh water, based on the entropy of mixing of the salt. Two methods are commonly used to extract this energy : PRO (pressure retarded osmosis) and RED (reverse electro-dialysis). In the PRO approach, a semi-impermeable membrane separates the salty and fresh water, which the ions cannot penetrate. The water is thus moving through the membrane and an osmotic pressure drop, which can be as large as ~30 bars, builds up between the two sides of the membrane. This pressure drop is used to move a turbine and produce hydro-electric power. On the other hand, in the RED approach, the ions are transported under the salt gradient, but across cation-selective and anion-selective membranes. This selectivity induces a separation of anion and cation fluxes and thus produces directly an electric current from the difference of salinity. Both approaches are now well established but the harvested power is typically of order of a few Watts per meter squared of membranes. It is crucial to explore new “out of the box” fundamental science in order to solve the current technological bottlenecks, which industries currently face to make this renewable energy viable.
Thanks to a new hierarchical nanofluidics set-up made by an individual transmembrane nanotube we have shown that nanofluidics can boost the efficiency uo to more than two orders of magnitude, making possible, and highly competitive, to harvest the chemical energy contained in the difference of salinity between sea water and river water.
Charges in a temperature gradient: Thermo-electro-osmosis and related effects
Alois Würger
Université de Bordeaux, France
In recent years temperature gradients have became a versatile means of actuation in colloidal suspensions. Thus thermophoresis has been used for sieving macromolecules and for dynamic particle confinement. On the other hand, laser heating of metal-capped Janus particles results in self-propulsion and orientational polarization.
The underlying mecanisms are not well understood, even for systems where charge effects dominate and where dispersion forces play a minor role. Indeed, most relevant parameters depend on temperature (Debye length, salinity, permittivity, thermoelectricity), thus contribute to the thermodynamic electric-double layer forces, and in some cases partly cancel each other.
In this contribution we discuss relevant effects in the double layer (Ruckenstein's thermoosmosis, the permittivity gradient, diffusiophoresis in a salinity gradient, the Seebeck effect). In particular we address specific-ion effects revealed by several recent experiments, and dicuss open questions.
- [1] A. Würger, Phys. Rev. Lett. 101, 108302 (2008); Rep. Prog. Phys. 73 126601 (2010)
- [2] A. Majee, A. Würger, Phys. Rev. Lett. 108 118301 (2012)
- [3] K.A. Eslahian, A. Majee, M. Maskos, A. Würger, Soft Matter (2014) arXiv:1401.7242

